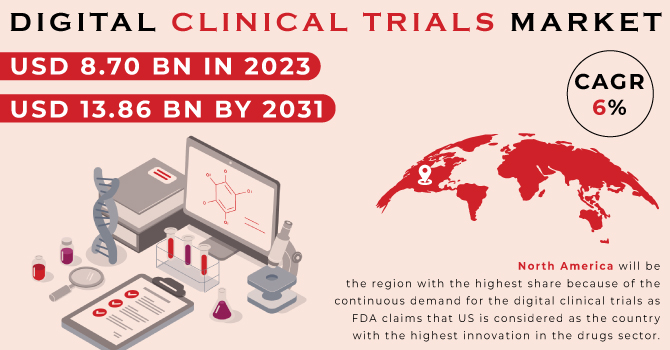
The Digital Clinical Trials industry that has seen transformative growth and innovation, was valued at USD 8.70 billion in 2023. According to recent market analysis, Digital Clinical Trials market size is anticipated to reach USD 13.86 billion by 2031, expanding at a Compound Annual Growth Rate (CAGR) of 6% over the forecast period from 2024 to 2031. This significant growth reflects the increasing adoption of digital technologies in clinical research and development.
A Revolutionary Shift in Clinical Trials
The traditional landscape of clinical trials has been fundamentally altered by the integration of digital technologies. Digital clinical trials, also known as virtual or decentralized clinical trials, leverage digital tools and platforms to conduct all or part of the research remotely. This paradigm shift not only enhances efficiency and accessibility but also accelerates the pace of clinical research.
Digital clinical trials utilize various technologies such as telemedicine, mobile health (mHealth) applications, electronic data capture (EDC), wearable devices, and remote monitoring tools. These innovations facilitate real-time data collection, improve patient engagement, and reduce the need for physical site visits, thereby making the clinical trial process more flexible and patient-centric.
Get a Free Sample Report of Digital Clinical Trials Market: https://www.snsinsider.com/sample-request/2690
Key Drivers of Market Growth
Several factors are driving the robust growth of the digital clinical trials market:
- Technological Advancements: The rapid evolution of digital health technologies, including AI and machine learning, is enhancing the capabilities of digital clinical trials. These technologies enable more precise data analysis, predictive modeling, and personalized medicine approaches.
- Increased Patient Engagement: Digital tools improve patient recruitment, retention, and compliance by providing a more convenient and user-friendly experience. Patients can participate in trials from the comfort of their homes, reducing travel burden and associated costs.
- Regulatory Support: Regulatory bodies are increasingly recognizing the benefits of digital clinical trials and are providing guidelines to ensure their safe and effective implementation. This regulatory support is crucial for the widespread adoption of digital methodologies in clinical research.
- Cost Efficiency: Digital clinical trials can significantly reduce operational costs by minimizing the need for physical infrastructure and streamlining data management processes. This cost efficiency is particularly beneficial for small and medium-sized enterprises (SMEs) in the pharmaceutical and biotechnology sectors.
- COVID-19 Pandemic: The COVID-19 pandemic has acted as a catalyst for the adoption of digital clinical trials. The necessity for remote monitoring and virtual interactions during the pandemic highlighted the value of digital solutions in maintaining continuity in clinical research.
Market Segmentation and Regional Insights
The digital clinical trials market can be segmented based on study type, end-user, and region.
- Study Type: The market includes interventional, observational, and expanded access studies. Interventional studies hold the largest market share due to their widespread use in testing new treatments and interventions.
- End-User: Pharmaceutical companies, biotechnology firms, and contract research organizations (CROs) are the primary end-users of digital clinical trials. Pharmaceutical companies are the dominant segment, driven by the need for efficient and cost-effective research and development processes.
- Region: North America leads the market, attributed to its advanced healthcare infrastructure, high adoption of digital technologies, and supportive regulatory environment. Europe and the Asia-Pacific region are also experiencing significant growth, driven by increasing healthcare investments and technological advancements.
Challenges and Opportunities
While the digital clinical trials market presents numerous opportunities, it also faces challenges that need to be addressed:
- Data Security and Privacy: Ensuring the security and privacy of patient data is paramount. Robust cybersecurity measures and compliance with data protection regulations are essential to maintain patient trust and integrity of the research.
- Technological Barriers: The successful implementation of digital clinical trials requires advanced technological infrastructure and expertise. Overcoming these barriers, especially in low-resource settings, is crucial for widespread adoption.
- Patient Diversity: Ensuring diverse and representative patient participation in digital clinical trials is a challenge. Strategies to reach underserved populations and address digital divides are necessary to achieve inclusive research outcomes.
Despite these challenges, the digital clinical trials market offers substantial opportunities for innovation and growth. The continuous advancement of digital health technologies, coupled with increasing industry collaborations, is expected to drive further progress in this field.
Future Outlook
The future of the digital clinical trials market looks promising, with continued advancements in technology and increasing acceptance of digital methodologies. As the industry evolves, stakeholders are likely to focus on integrating more sophisticated tools such as blockchain for data integrity, AI for predictive analytics, and real-world evidence (RWE) for more comprehensive insights.
Moreover, patient-centric approaches will remain at the forefront, with efforts to enhance patient engagement and experience through user-friendly digital platforms and personalized interventions. The growing emphasis on patient-reported outcomes (PROs) and real-time data collection will further enrich clinical research and facilitate more informed decision-making.
Conclusion
In conclusion, the digital clinical trials market is set to witness significant growth over the next decade, driven by technological innovations, regulatory support, and increasing demand for efficient and patient-centric research methodologies. As the market expands, it will play a crucial role in accelerating the development of new treatments and improving patient outcomes, ultimately transforming the landscape of clinical research.
Other Reports You May Like:
Digital Hearing Aids Market Size
Cognitive Behavioral Therapy Market Size

